Atossa Genetics Inc. (ATOS): Price and Financial Metrics
ATOS Price/Volume Stats
| Current price | $1.45 | 52-week high | $2.31 |
| Prev. close | $1.42 | 52-week low | $0.59 |
| Day low | $1.45 | Volume | 14,608 |
| Day high | $1.45 | Avg. volume | 1,381,663 |
| 50-day MA | $1.39 | Dividend yield | N/A |
| 200-day MA | $0.98 | Market Cap | 181.69M |
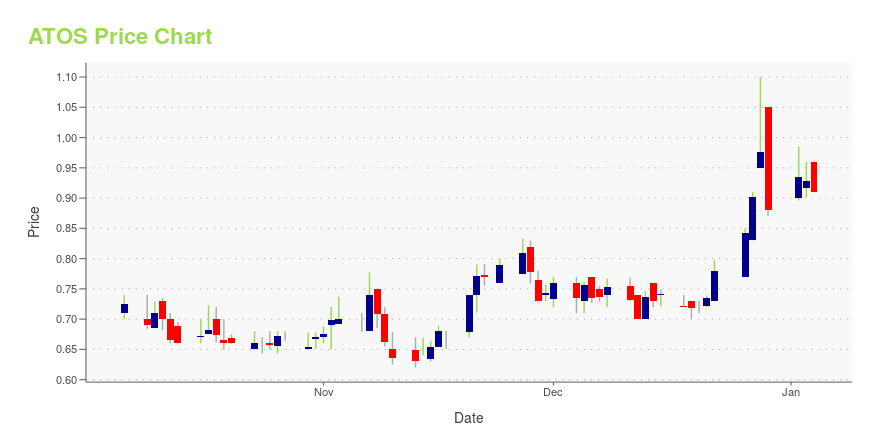
ATOS Stock Price Chart Interactive Chart >
Atossa Genetics Inc. (ATOS) Company Bio
Atossa Therapeutics, Inc. develops and markets medical devices, laboratory tests, and therapeutics to address breast health conditions in the United States. The company's lead program is the development of Endoxifen, an active metabolite of tamoxifen which is in Phase II studies to treat and prevent breast cancer. It is also developing intraductal microcatheter technology to target the delivery of therapies, including fulvestrant, immunotherapies, and Chimeric Antigen Receptor T-cell therapies, directly to the site of breast cancer. The company was formerly known as Atossa Genetics Inc. and changed its name to Atossa Therapeutics, Inc. in January 2020. Atossa Therapeutics, Inc. was founded in 2009 and is headquartered in Seattle, Washington.
Latest ATOS News From Around the Web
Below are the latest news stories about ATOSSA THERAPEUTICS INC that investors may wish to consider to help them evaluate ATOS as an investment opportunity.
Is Atossa Genetics (ATOS) Outperforming Other Medical Stocks This Year?Here is how Atossa Genetics Inc. (ATOS) and Amphastar Pharmaceuticals (AMPH) have performed compared to their sector so far this year. |
Atossa Therapeutics Announces the Appointment of Arezoo Mirad, M.D., as Senior Medical DirectorSEATTLE, Dec. 04, 2023 (GLOBE NEWSWIRE) -- Atossa Therapeutics, Inc. (Nasdaq: ATOS), a clinical stage biopharmaceutical company developing innovative medicines in areas of significant unmet medical need in oncology with a focus on breast cancer, today announced the appointment of Dr. Arezoo Mirad as Senior Medical Director. “We are excited to welcome Dr. Mirad to Atossa and look forward to leveraging her extensive experience in the clinical development of oncology candidates to further accelerat |
Atossa Therapeutics Announces Full Enrollment of Phase 2 Karisma-Endoxifen Clinical TrialSEATTLE, Nov. 20, 2023 (GLOBE NEWSWIRE) -- Atossa Therapeutics, Inc. (Nasdaq: ATOS), a clinical stage biopharmaceutical company developing innovative medicines in areas of significant unmet medical need in oncology with a focus on breast cancer, today announced that it has reached full enrollment in the Company’s Karisma-Endoxifen clinical trial, the 240-person Phase 2 study investigating (Z)-endoxifen in premenopausal women with measurable mammographic breast density (MBD). Participants have be |
Atossa Therapeutics Announces Third Quarter 2023 Financial Results and Provides Corporate UpdateAchieved significant enrollment milestones in ongoing Phase 2 clinical trialsFour ongoing Phase 2 studies evaluating (Z)-endoxifen, including recently announced study in DCIS Ended third quarter 2023 with $94.0 million of cash and cash equivalents SEATTLE, Nov. 13, 2023 (GLOBE NEWSWIRE) -- Atossa Therapeutics, Inc. (Nasdaq: ATOS), a clinical stage biopharmaceutical company developing innovative proprietary medicines to address significant unmet needs in oncology with a focus on breast cancer, to |
Atossa Therapeutics Appoints Financial Executive Jonathan Finn to its Board of DirectorsSEATTLE, Nov. 09, 2023 (GLOBE NEWSWIRE) -- Atossa Therapeutics, Inc. (Nasdaq: ATOS), a clinical stage biopharmaceutical company developing innovative medicines in areas of significant unmet medical need in oncology with a focus on breast cancer, today announced that Jonathan Finn, CFA, has been appointed to Atossa’s board of directors, effective November 8, 2023. Mr. Finn has more than 25 years of experience in the financial industry with a focus on early to mid-stage biotech and technology comp |
ATOS Price Returns
| 1-mo | -13.17% |
| 3-mo | 79.23% |
| 6-mo | 115.87% |
| 1-year | 115.77% |
| 3-year | -18.08% |
| 5-year | -54.69% |
| YTD | 64.77% |
| 2023 | 66.51% |
| 2022 | -66.97% |
| 2021 | 68.42% |
| 2020 | -39.49% |
| 2019 | 53.92% |

Loading social stream, please wait...