bluebird bio, Inc. (BLUE): Price and Financial Metrics
BLUE Price/Volume Stats
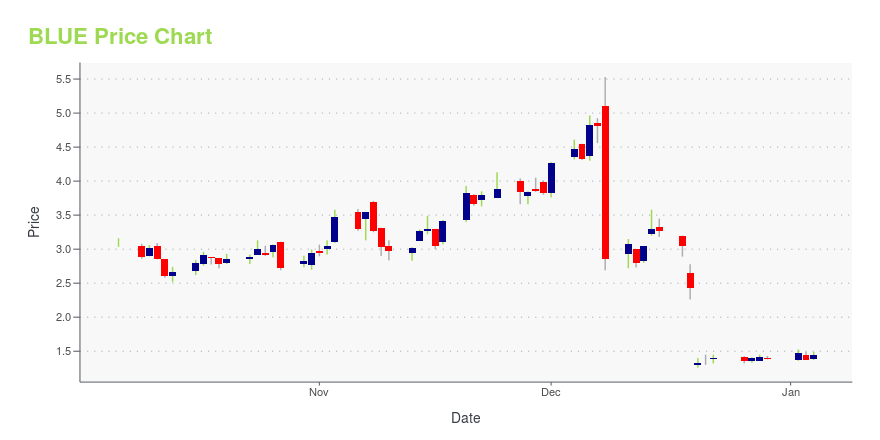
| Current price | $0.95 | 52-week high | $5.53 |
| Prev. close | $1.02 | 52-week low | $0.88 |
| Day low | $0.94 | Volume | 4,581,900 |
| Day high | $1.03 | Avg. volume | 8,894,379 |
| 50-day MA | $1.26 | Dividend yield | N/A |
| 200-day MA | $2.50 | Market Cap | 104.26M |
BLUE Stock Price Chart Interactive Chart >
bluebird bio, Inc. (BLUE) Company Bio
Bluebird Bio is a clinical-stage company committed to developing potentially transformative gene therapies for severe genetic and rare diseases and T cell-based immunotherapies. The company was founded in 1992 and is based in Cambridge, Massachusetts.
Latest BLUE News From Around the Web
Below are the latest news stories about BLUEBIRD BIO INC that investors may wish to consider to help them evaluate BLUE as an investment opportunity.
Should You Invest in Bluebird Bio Right Now?After two December share price crashes, there's no evident cure for Bluebird Bio's ills. |
If You Invested $10,000 in Bluebird Bio In 2021, This Is How Much You Would Have TodayCan things get better for this struggling stock? |
1 Green Flag and 1 Red Flag for Bluebird Bio StockThe stock has been in the news lately. |
3 Money-Saving Tricks for Investing in Biotech Stocks in 2024Doing a little math and doing a little extra research can make a big difference. |
Bluebird Bio Stock Is in Free Fall Weeks After a Landmark FDA Approval. Here’s Why.The plummeting share price is a potent symbol of the structural problems still facing small and midsize biotechs. |
BLUE Price Returns
| 1-mo | -30.66% |
| 3-mo | -11.21% |
| 6-mo | -68.95% |
| 1-year | -72.38% |
| 3-year | -96.68% |
| 5-year | -99.32% |
| YTD | -31.16% |
| 2023 | -80.06% |
| 2022 | -30.73% |
| 2021 | -76.91% |
| 2020 | -50.69% |
| 2019 | -11.54% |
Continue Researching BLUE
Want to do more research on bluebird bio Inc's stock and its price? Try the links below:bluebird bio Inc (BLUE) Stock Price | Nasdaq
bluebird bio Inc (BLUE) Stock Quote, History and News - Yahoo Finance
bluebird bio Inc (BLUE) Stock Price and Basic Information | MarketWatch

Loading social stream, please wait...