Daré Bioscience, Inc. (DARE): Price and Financial Metrics
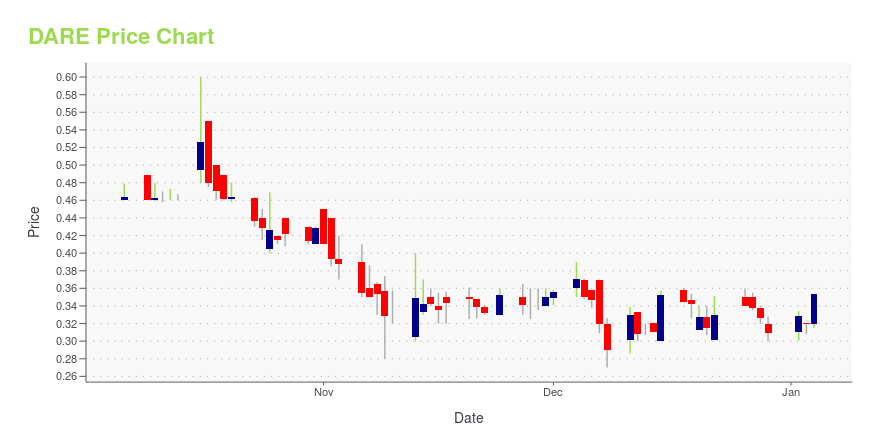
DARE Price/Volume Stats
| Current price | $0.48 | 52-week high | $1.10 |
| Prev. close | $0.47 | 52-week low | $0.27 |
| Day low | $0.46 | Volume | 204,500 |
| Day high | $0.48 | Avg. volume | 382,767 |
| 50-day MA | $0.46 | Dividend yield | N/A |
| 200-day MA | $0.50 | Market Cap | 48.29M |
DARE Stock Price Chart Interactive Chart >
Daré Bioscience, Inc. (DARE) Company Bio
Daré Bioscience, Inc., a clinical-stage biopharmaceutical company, focuses on developing and marketing products for women's health in the United States. The company intends to develop therapies in the areas of contraception, fertility, and sexual and vaginal health. Its products in advanced clinical development include DARE-BV1, a bioadhesive hydrogel formulated with clindamycin phosphate 2% to treat bacterial vaginosis in a single application that completed Phase 3 clinical trials; Ovaprene, a hormone-free, monthly vaginal contraceptive; and Sildenafil Cream, a cream formulation of sildenafil for topical administration to the vulva and vagina for treatment of female sexual arousal disorder. The company's Phase 1-ready products are DARE-HRT1, a combination of bio-identical estradiol and progesterone intravaginal ring for the treatment of vasomotor symptoms in hormone replacement therapy; DARE-VVA1, a vaginally delivered formulation of tamoxifen to treat vulvar vaginal atrophy in patients with hormone-receptor positive breast cancer; and DARE-FRT1, an intravaginal ring containing bio-identical progesterone for the prevention of preterm birth and broader luteal phase support as part of an in vitro fertilization treatment plan. Its products in pre-clinical stage include DARE-LARC1, a combination product designed to provide reversible contraception; ORB-204 and ORB-214 injectable formulations of etonogestrel to provide contraception over 6-month and 12-month periods; and DARE-RH1, a non-hormonal contraception for men and women. The company is based in San Diego, California.
Latest DARE News From Around the Web
Below are the latest news stories about DARE BIOSCIENCE INC that investors may wish to consider to help them evaluate DARE as an investment opportunity.
Daré Bioscience Secures $12 million in Royalty-backed Investment Structure$5 million at closing and up to $7 million of additional committed fundingSAN DIEGO, Dec. 26, 2023 (GLOBE NEWSWIRE) -- Daré Bioscience, Inc. (NASDAQ: DARE), a leader in women’s health innovation, today announced it has entered into a royalty-backed financing agreement that provides Daré with $5 million at closing and up to an additional $7 million through three tranches over time at Daré’s option. The financing structure entitles the investor to a percentage of the royalties and milestones to be |
Daré Bioscience Announces Positive Topline Pharmacokinetic and Exploratory Efficacy Results from the DARE-PDM1 Phase 1 Clinical StudyData Support Continued Clinical Development of DARE-PDM1 and its Potential as a First-in-Category Treatment for Primary DysmenorrheaSAN DIEGO, Dec. 20, 2023 (GLOBE NEWSWIRE) -- Daré Bioscience, Inc. (NASDAQ: DARE), a leader in women’s health innovation, today announced positive topline results from the Phase 1 study evaluating the pharmacokinetics (PK), safety, and exploratory efficacy of DARE-PDM1. DARE-PDM1 is an investigational product designed to deliver diclofenac, a nonsteroidal anti-infl |
Daré Bioscience Announces Funding Award Notice from the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to Support DARE-PTB1 Phase 1 Human Clinical StudyDARE-PTB1 is being developed as a treatment to reduce the risk of preterm birth, for which there are no FDA-approved treatment options Up to approximately $2 million in grant funding to support DARE-PTB1 Phase 1 human clinical study SAN DIEGO, Dec. 13, 2023 (GLOBE NEWSWIRE) -- Daré Bioscience, Inc. (NASDAQ: DARE), a leader in women’s health innovation, today announced that it received a Notice of Award of a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Develo |
Daré Bioscience Announces FDA Clearance of Investigational New Drug (IND) Application for DARE-VVA1, a Novel Intravaginal Formulation of Tamoxifen for Moderate to Severe Dyspareunia, a Symptom of Vulvar and Vaginal Atrophy Associated with MenopauseDARE-VVA1 has the potential to be the first therapeutic non-hormonal vaginal option for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy (VVA) in women who cannot or should not take supplemental estrogenSAN DIEGO, Dec. 07, 2023 (GLOBE NEWSWIRE) -- Daré Bioscience, Inc. (NASDAQ:DARE), a leader in women’s health innovation, today announced that the U.S. Food and Drug Administration (FDA) has cleared its investigational new drug (IND) application for DARE-VVA |
Daré Bioscience Announces Commencement of Phase 3 Study of Ovaprene®, an Investigational Hormone-Free Monthly Intravaginal ContraceptiveOvaprene has the Potential to be the First FDA-Approved Monthly, Self-Administered, Hormone-Free Contraceptive ProductSAN DIEGO, Dec. 04, 2023 (GLOBE NEWSWIRE) -- Daré Bioscience, Inc. (NASDAQ: DARE), a leader in women’s health innovation, today announced commencement of the Company’s pivotal Phase 3 clinical study of Ovaprene, an investigational hormone-free monthly intravaginal contraceptive. The multi-center, single arm, non-comparative, pivotal Phase 3 clinical study of Ovaprene will evaluat |
DARE Price Returns
| 1-mo | 2.48% |
| 3-mo | 51.37% |
| 6-mo | 4.01% |
| 1-year | -52.94% |
| 3-year | -65.71% |
| 5-year | -52.48% |
| YTD | 55.34% |
| 2023 | -62.77% |
| 2022 | -58.50% |
| 2021 | 49.25% |
| 2020 | 63.41% |
| 2019 | 15.49% |

Loading social stream, please wait...