Immunocore Holdings plc (IMCR): Price and Financial Metrics
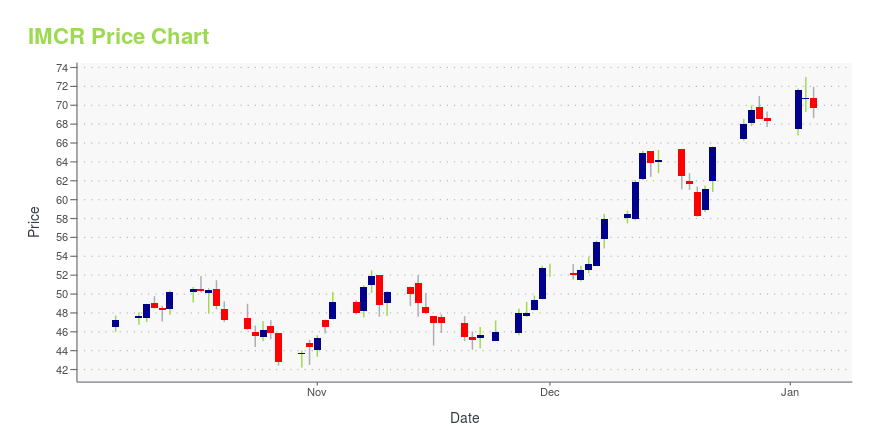
IMCR Price/Volume Stats
| Current price | $57.31 | 52-week high | $76.98 |
| Prev. close | $55.82 | 52-week low | $42.21 |
| Day low | $56.25 | Volume | 302,100 |
| Day high | $57.98 | Avg. volume | 484,333 |
| 50-day MA | $63.31 | Dividend yield | N/A |
| 200-day MA | $59.70 | Market Cap | 2.86B |
IMCR Stock Price Chart Interactive Chart >
Immunocore Holdings plc (IMCR) Company Bio
Immunocore Holdings Limited operates as a holding company. The Company, through its subsidiaries, develops and commercializes a new generation of transformative medicines to address unmet needs in cancer, infection, and autoimmune diseases. Immunocore Holdings serves customers in the United Kingdom and the United States.
Latest IMCR News From Around the Web
Below are the latest news stories about IMMUNOCORE HOLDINGS PLC that investors may wish to consider to help them evaluate IMCR as an investment opportunity.
Immunocore Holdings PLC Sponsored ADR (IMCR) Upgraded to Buy: Here's What You Should KnowImmunocore Holdings PLC Sponsored ADR (IMCR) has been upgraded to a Zacks Rank #2 (Buy), reflecting growing optimism about the company's earnings prospects. This might drive the stock higher in the near term. |
How Much Upside is Left in Immunocore Holdings PLC Sponsored ADR (IMCR)? Wall Street Analysts Think 30.32%The consensus price target hints at a 30.3% upside potential for Immunocore Holdings PLC Sponsored ADR (IMCR). While empirical research shows that this sought-after metric is hardly effective, an upward trend in earnings estimate revisions could mean that the stock will witness an upside in the near term. |
Levicept Appoints Eliot Forster as CEOLeadership Team Strengthened as Clinical Development of LEVI-04 a Novel Neurotrophin Modulator for Osteoarthritis and Chronic Pain Advances SANDWICH, United Kingdom, Nov. 30, 2023 (GLOBE NEWSWIRE) -- Levicept Ltd, a biotechnology company focused on the development of LEVI-04, a first-in-class treatment for chronic pain indications, today announces the appointment of Eliot Forster as CEO. Founder and inventor of LEVI-04, Simon Westbrook, is to take the role of CSO. Eliot brings more than thirty y |
Can Immunocore Holdings PLC Sponsored ADR (IMCR) Climb 75.55% to Reach the Level Wall Street Analysts Expect?The consensus price target hints at a 75.6% upside potential for Immunocore Holdings PLC Sponsored ADR (IMCR). While empirical research shows that this sought-after metric is hardly effective, an upward trend in earnings estimate revisions could mean that the stock will witness an upside in the near term. |
Bears are Losing Control Over Immunocore Holdings PLC Sponsored ADR (IMCR), Here's Why It's a 'Buy' NowImmunocore Holdings PLC Sponsored ADR (IMCR) witnesses a hammer chart pattern, indicating support found by the stock after losing some value lately. This coupled with an upward trend in earnings estimate revisions could mean a trend reversal for the stock in the near term. |
IMCR Price Returns
| 1-mo | -8.33% |
| 3-mo | -20.04% |
| 6-mo | 33.75% |
| 1-year | -5.62% |
| 3-year | 52.58% |
| 5-year | N/A |
| YTD | -16.12% |
| 2023 | 19.71% |
| 2022 | 66.68% |
| 2021 | N/A |
| 2020 | N/A |
| 2019 | N/A |

Loading social stream, please wait...