Processa Pharmaceuticals, Inc. (PCSA): Price and Financial Metrics
PCSA Price/Volume Stats
| Current price | $1.58 | 52-week high | $18.00 |
| Prev. close | $1.56 | 52-week low | $1.40 |
| Day low | $1.51 | Volume | 19,700 |
| Day high | $1.62 | Avg. volume | 1,380,620 |
| 50-day MA | $2.25 | Dividend yield | N/A |
| 200-day MA | $6.21 | Market Cap | 4.51M |
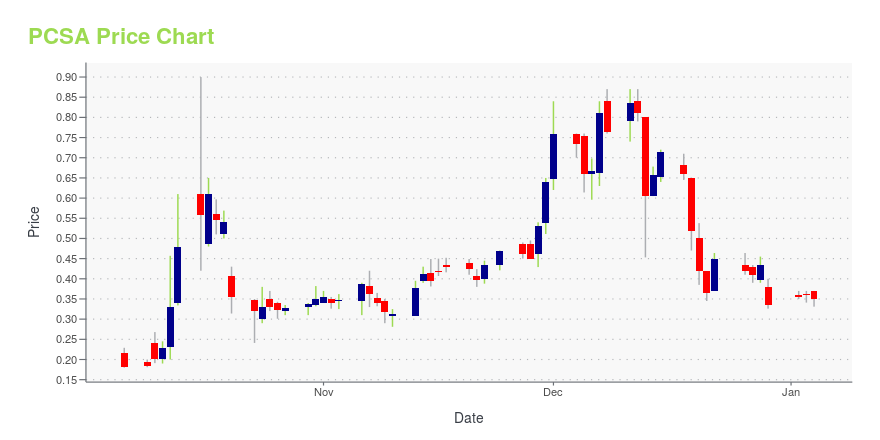
PCSA Stock Price Chart Interactive Chart >
Processa Pharmaceuticals, Inc. (PCSA) Company Bio
Processa Pharmaceuticals, Inc. operates as a pharmaceutical company. The Company focuses on development of drug products that are intended to improve the survival and quality of life for patients who have unmet medical need. Processa Pharmaceuticals serves patients in the United States.
Latest PCSA News From Around the Web
Below are the latest news stories about PROCESSA PHARMACEUTICALS INC that investors may wish to consider to help them evaluate PCSA as an investment opportunity.
Processa Pharmaceuticals Provides Interim Analysis from Ongoing Phase 1b Trial of Next Generation Capecitabine Showing Improved Safety Over CapecitabineFDA acknowledges that NGC-Cap is a New Chemical Entity given the changes to metabolism and distribution of its major metabolite 5-FU Interim analysis of the NGC-Cap Phase 1b data shows improved safety, even with 5-FU exposure much greater than that from capecitabine Company to conduct Fireside Chat at 4:30PM ET on December 20, 2023 HANOVER, MD, Dec. 19, 2023 (GLOBE NEWSWIRE) -- Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) (“Processa” or the “Company”), a clinical-stage pharmaceutical company fo |
Processa Pharmaceuticals Announces Successful Phase 2 Meeting with FDA for Next Generation CapecitabineFDA provides guidance on Phase 2 design and Processa to continue with pre-study activities for Phase 2 trial HANOVER, MD, Dec. 13, 2023 (GLOBE NEWSWIRE) -- Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) (“Processa” or the “Company”), a clinical-stage pharmaceutical company focused on developing the next generation of chemotherapeutic drugs to improve the efficacy and safety for more patients suffering from cancer, announces the outcomes from a successful meeting with the U.S. Food and Drug Admini |
Is Processa Pharmaceuticals (NASDAQ:PCSA) In A Good Position To Invest In Growth?Just because a business does not make any money, does not mean that the stock will go down. For example, biotech and... |
Processa Pharmaceuticals to Present at the MedInvest Oncology Investor ConferenceHANOVER, MD, Nov. 30, 2023 (GLOBE NEWSWIRE) -- Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) (“Processa” or the “Company”), a clinical-stage pharmaceutical company focused on developing the next generation of chemotherapeutic drugs to improve the efficacy and safety for patients suffering from cancer, announces that Dr. David Young, President of Research and Development, will present virtually at the MedInvest Oncology Investor Conference. The conference is being held on December 5 – 6, 2023 in |
Processa Pharmaceuticals Issues Letter to Shareholders Highlighting Corporate Strategy, Drug Pipeline, and OutlookHANOVER, MD, Nov. 29, 2023 (GLOBE NEWSWIRE) -- Processa Pharmaceuticals, Inc. (Nasdaq: PCSA) (“Processa” or the “Company”), a clinical-stage pharmaceutical company now focused on developing the next generation of chemotherapeutic drugs to improve the efficacy and safety for patients suffering from cancer, announces that CEO George Ng has issued a letter to shareholders. Dear Processa Pharmaceuticals Shareholders: Following Processa’s business pivot to focus on oncology drug product development, |
PCSA Price Returns
| 1-mo | -27.19% |
| 3-mo | -64.89% |
| 6-mo | -75.31% |
| 1-year | -85.11% |
| 3-year | -99.03% |
| 5-year | N/A |
| YTD | -76.39% |
| 2023 | -69.58% |
| 2022 | -77.55% |
| 2021 | -25.70% |
| 2020 | N/A |
| 2019 | 0.00% |

Loading social stream, please wait...