Roivant Sciences Ltd. (ROIV): Price and Financial Metrics
ROIV Price/Volume Stats
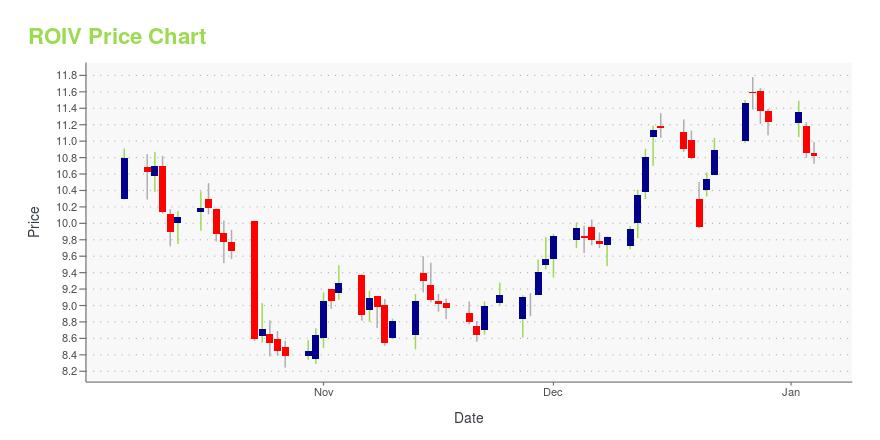
| Current price | $10.94 | 52-week high | $13.24 |
| Prev. close | $10.60 | 52-week low | $8.06 |
| Day low | $10.61 | Volume | 4,978,800 |
| Day high | $11.05 | Avg. volume | 6,865,613 |
| 50-day MA | $10.94 | Dividend yield | N/A |
| 200-day MA | $10.64 | Market Cap | 8.82B |
ROIV Stock Price Chart Interactive Chart >
Roivant Sciences Ltd. (ROIV) Company Bio
Roivant Sciences Ltd., a biopharmaceutical and healthcare technology company that researches and develops medicines. The company develops product candidates for the treatment of various therapeutics, including solid tumors, sickle cell diseases, hypophosphatasia, oncologic malignancies, psoriasis, atopic dermatitis, vitiligo, hyperhidrosis, acne, myasthenia gravis, warm autoimmune hemolytic anemia, thyroid eye diseases, sarcoidosis, and staph aureus bacteremia. The company was founded in 2014 and is based in London, the United Kingdom.
Latest ROIV News From Around the Web
Below are the latest news stories about ROIVANT SCIENCES LTD that investors may wish to consider to help them evaluate ROIV as an investment opportunity.
Roivant Reports Positive Initial Phase 2 Results for Batoclimab in Graves’ DiseaseNEW YORK, Dec. 20, 2023 (GLOBE NEWSWIRE) -- Immunovant, Inc. (Nasdaq: IMVT), a clinical-stage immunology company dedicated to enabling normal lives for people with autoimmune diseases, today announced that results from the initial cohort of patients in an ongoing 24-week Phase 2 clinical trial of batoclimab in patients with Graves’ disease meaningfully exceeded 50% response rates. This Phase 2 proof-of-concept trial is an open-label study to assess the safety and efficacy of batoclimab in Graves |
Roche Completes Acquisition of Telavant from Roivant, Including Rights to Novel TL1A Directed Antibody (RVT-3101) for the Treatment of Inflammatory Bowel DiseaseBASEL, Switzerland and LONDON and NEW YORK, Dec. 14, 2023 (GLOBE NEWSWIRE) -- Roivant (Nasdaq: ROIV) announced today the completion of the previously announced acquisition by Roche (SIX: RO, ROG; OTCQX: RHHBY) of Telavant, for an upfront payment of approximately $7.1 billion. Telavant holds the rights in the US and Japan to RVT-3101, a promising new therapy in development for people suffering from inflammatory bowel disease, including ulcerative colitis and Crohn’s disease. Prior to the closing |
Roivant Announces Positive IMVT-1402 Initial 600 mg MAD Results that Confirm Best-in-Class PotentialResults from the 600 mg MAD cohort for IMVT-1402 similar to previously disclosed results from the 300 mg MAD cohort for IMVT-1402IMVT-1402 was observed to deliver dose dependent and deep IgG reductions similar to batoclimab in its Phase 1 studyIMVT-1402 600 mg was observed to deliver placebo-like impact on albumin and low-density lipoprotein cholesterol (LDL-C), similar to the previously disclosed 300 mg MAD cohort dataPotential best-in-class profile enables broad and exciting portfolio of indic |
Roivant and Priovant Announce Results from Phase 2 Study of Oral Brepocitinib in Systemic Lupus ErythematosusOral brepocitinib failed to meet its primary endpoint of Systemic Lupus Erythematosus Responder Index (SRI-4) change of 4 at Week 52Priovant plans to continue progressing the program in indications outside of Systemic Lupus Erythematosus (SLE) given the drug’s favorable safety and tolerability profile, six other positive phase 2 studies, and active arm performance in this studyPriovant expects to announce topline results from the Phase 2 POC study of brepocitinib in non-infectious uveitis (NIU) |
Roivant Reports Financial Results for the Second Quarter Ended September 30, 2023, and Provides Business UpdateRoivant entered into a definitive agreement with Roche for the sale of Telavant for $7.1B upfront and a milestone payment of $150M payable upon the initiation of a Phase 3 trial in ulcerative colitisIMVT-1402 subcutaneous (SC) doses achieved peak Immunoglobulin G (IgG) reductions that are similar to those previously observed with batoclimab, based on initial results from Phase 1 single-ascending dose and 300 mg multiple-ascending dose studiesIMVT-1402 showed no statistically significant dose-rel |
ROIV Price Returns
| 1-mo | 7.78% |
| 3-mo | 5.19% |
| 6-mo | 29.47% |
| 1-year | 23.34% |
| 3-year | N/A |
| 5-year | N/A |
| YTD | -2.58% |
| 2023 | 40.55% |
| 2022 | -20.73% |
| 2021 | N/A |
| 2020 | N/A |
| 2019 | N/A |

Loading social stream, please wait...