Aldeyra Therapeutics, Inc. (ALDX): Price and Financial Metrics
ALDX Price/Volume Stats
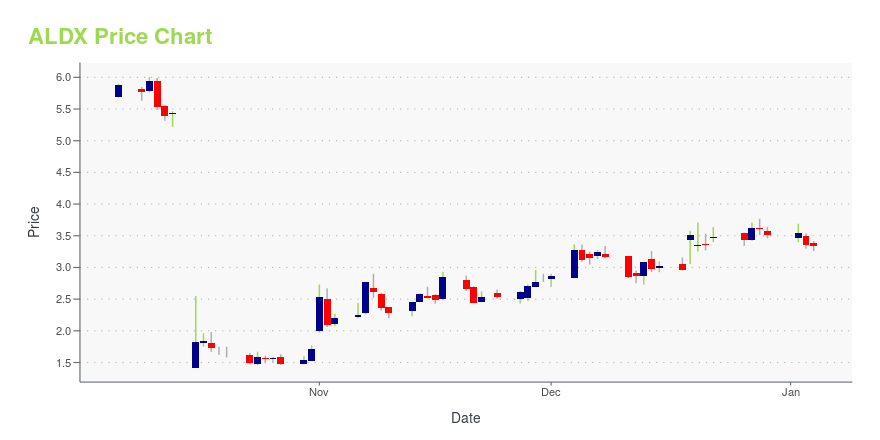
| Current price | $4.15 | 52-week high | $8.38 |
| Prev. close | $4.09 | 52-week low | $1.42 |
| Day low | $4.08 | Volume | 200,009 |
| Day high | $4.21 | Avg. volume | 471,494 |
| 50-day MA | $3.74 | Dividend yield | N/A |
| 200-day MA | $3.39 | Market Cap | 246.57M |
ALDX Stock Price Chart Interactive Chart >
Aldeyra Therapeutics, Inc. (ALDX) Company Bio
Aldeyra Therapeutics, Inc., a biotechnology company, focuses on the development of products for diseases caused by inflammation and inborn errors of metabolism in the United States and internationally. The company was formerly known as Aldexa Therapeutics, Inc. and changed its name to Aldeyra Therapeutics, Inc. in March 2014. Aldeyra Therapeutics, Inc. was founded in 2004 and is based in Lexington, Massachusetts.
Latest ALDX News From Around the Web
Below are the latest news stories about ALDEYRA THERAPEUTICS INC that investors may wish to consider to help them evaluate ALDX as an investment opportunity.
Aldeyra Therapeutics Announces Statistically and Clinically Significant Improvement from Baseline in Phase 2 Clinical Trial of ADX‑629 in Patients with Atopic DermatitisLEXINGTON, Mass., December 19, 2023--Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated diseases, today announced positive top-line results from a Phase 2 clinical trial of ADX-629, an investigational RASP modulator, in patients with atopic dermatitis. Relative to baseline, the clinical trial demonstrated statistically significant and clinically relevant improvement in investiga |
Aldeyra Therapeutics Schedules Conference Call and Webcast to Announce Top-Line Results from Phase 2 Clinical Trial of ADX-629 in Patients with Atopic DermatitisLEXINGTON, Mass., December 18, 2023--Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra) today announced it will host a webcast and conference call on Tuesday, December 19, 2023, at 8:00 a.m. (ET) to provide top-line results from a Phase 2 clinical trial of ADX‑629 in patients with atopic dermatitis. |
13 Hot Penny Stocks To Buy According to Hedge FundsIn this article, we will take a look at the 13 Hot Penny Stocks To Buy According to Hedge Funds. To see more hot penny stocks, go directly to 5 Hot Penny Stocks To Buy According to Hedge Funds. The overall market optimism helped by expectations that the Fed might start cutting interest rates in […] |
3 Stocks at the Forefront of Personalized Medicine TrendUnveiling the impact of personalized medicine stocks, transforming patient care, and propelling market growth seamlessly |
Semtech (SMTC) to Release Q3 Earnings: What's in the Cards?Semtech's (SMTC) third-quarter fiscal 2024 results are expected to reflect solid momentum in the industrial market amid macroeconomic challenges. |
ALDX Price Returns
| 1-mo | 27.69% |
| 3-mo | 5.60% |
| 6-mo | 36.51% |
| 1-year | -45.68% |
| 3-year | -52.89% |
| 5-year | -22.43% |
| YTD | 18.23% |
| 2023 | -49.57% |
| 2022 | 74.00% |
| 2021 | -41.69% |
| 2020 | 18.07% |
| 2019 | -30.00% |

Loading social stream, please wait...