Avenue Therapeutics, Inc. (ATXI): Price and Financial Metrics
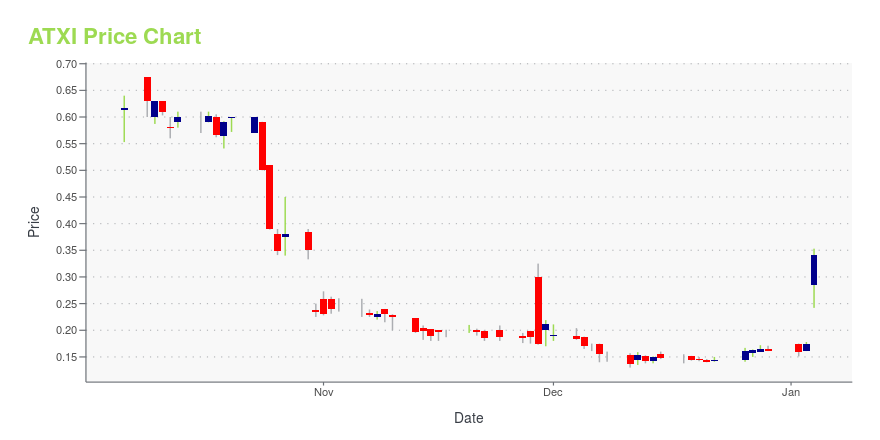
ATXI Price/Volume Stats
| Current price | $2.72 | 52-week high | $85.50 |
| Prev. close | $2.87 | 52-week low | $2.60 |
| Day low | $2.60 | Volume | 133,739 |
| Day high | $3.06 | Avg. volume | 70,259 |
| 50-day MA | $3.53 | Dividend yield | N/A |
| 200-day MA | $11.65 | Market Cap | 2.56M |
ATXI Stock Price Chart Interactive Chart >
Avenue Therapeutics, Inc. (ATXI) Company Bio
Avenue Therapeutics, Inc., a specialty pharmaceutical company, acquires, licenses, develops, and commercializes an intravenous formulation of tramadol HCI principally for use in the acute/intensive care hospital setting. Its product candidate is IV Tramadol for the treatment of post-operative pain. The company was founded in 2015 and is based in New York, New York.
Latest ATXI News From Around the Web
Below are the latest news stories about AVENUE THERAPEUTICS INC that investors may wish to consider to help them evaluate ATXI as an investment opportunity.
Avenue Therapeutics to Present at American Epilepsy Society 2023 Annual MeetingMIAMI, Dec. 01, 2023 (GLOBE NEWSWIRE) -- Avenue Therapeutics, Inc. (Nasdaq: ATXI) (“Avenue” or the “Company”), a specialty pharmaceutical company focused on the development and commercialization of therapies for the treatment of neurologic diseases, today announced that Amy Chappell, M.D., FAAN, will be presenting preclinical in vivo data evaluating BAER-101 using the SynapCell's Genetic Absence Epilepsy Rat from Strasbourg (“GAERS”) model of absence epilepsy at the American Epilepsy Society (AE |
Today’s Biggest Pre-Market Stock Movers: 10 Top Gainers and Losers on ThursdayIt's time for a breakdown of the biggest pre-market stock movers as we check out all of the hottest news on Thursday morning! |
Why Is Avenue Therapeutics (ATXI) Stock Up 59% Today?Avenue Therapeutics stock is rising higher on Wednesday following news of an investor taking a 14.5% stake in ATXI shares. |
Why Is Leslie’s (LESL) Stock Down 24% Today?Leslie's stock is falling hard on Wednesday as investors in LESL react to it missing EPS estimates in Q4 and posting weak 2024 guidance. |
Why Is Seelos Therapeutics (SEEL) Stock Down 42% Today?Seelos Therapeutics stock is falling on Wednesday after the company announced and priced a public offering for shares of SEEL. |
ATXI Price Returns
| 1-mo | -25.89% |
| 3-mo | -56.13% |
| 6-mo | -75.82% |
| 1-year | -96.55% |
| 3-year | -99.87% |
| 5-year | -99.96% |
| YTD | -77.47% |
| 2023 | -86.12% |
| 2022 | -91.48% |
| 2021 | -84.74% |
| 2020 | -38.02% |
| 2019 | 77.78% |

Loading social stream, please wait...