Calliditas Therapeutics AB ADR (CALT): Price and Financial Metrics
CALT Price/Volume Stats
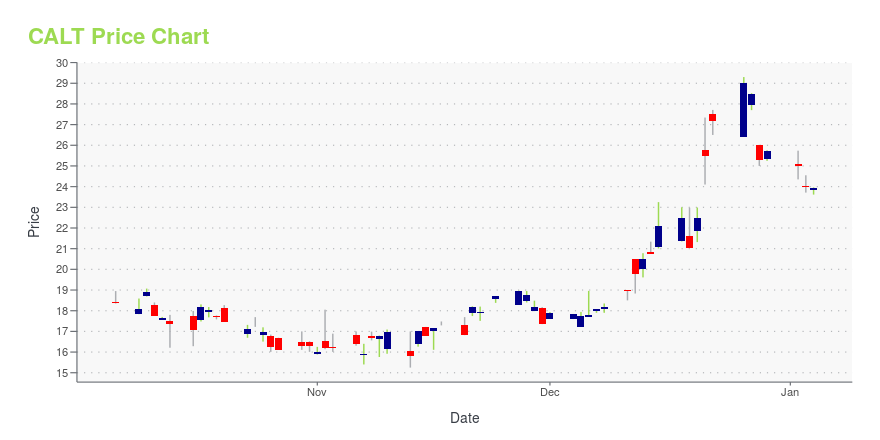
| Current price | $40.00 | 52-week high | $43.00 |
| Prev. close | $40.16 | 52-week low | $15.25 |
| Day low | $40.00 | Volume | 9,700 |
| Day high | $43.00 | Avg. volume | 10,339 |
| 50-day MA | $39.50 | Dividend yield | N/A |
| 200-day MA | $0.00 | Market Cap | 1.19B |
CALT Stock Price Chart Interactive Chart >
Calliditas Therapeutics AB ADR (CALT) Company Bio
Calliditas Therapeutics AB provides pharmaceutical products for patients with niche indications. It focuses on development and commercialization of the product candidate Nefecon intended for treatment of patients with the inflammatory renal disease IgA nephropathy. The company was founded by Mikael Bender and Bengt Åke Julander on February 20, 2004 and is headquartered in Stockholm, Sweden.
CALT Price Returns
| 1-mo | N/A |
| 3-mo | N/A |
| 6-mo | N/A |
| 1-year | 3.12% |
| 3-year | N/A |
| 5-year | 55.70% |
| YTD | N/A |
| 2024 | 0.00% |
| 2023 | 51.44% |
| 2022 | -31.34% |
| 2021 | -26.35% |
| 2020 | N/A |

Loading social stream, please wait...