Celularity Inc., (CELU): Price and Financial Metrics
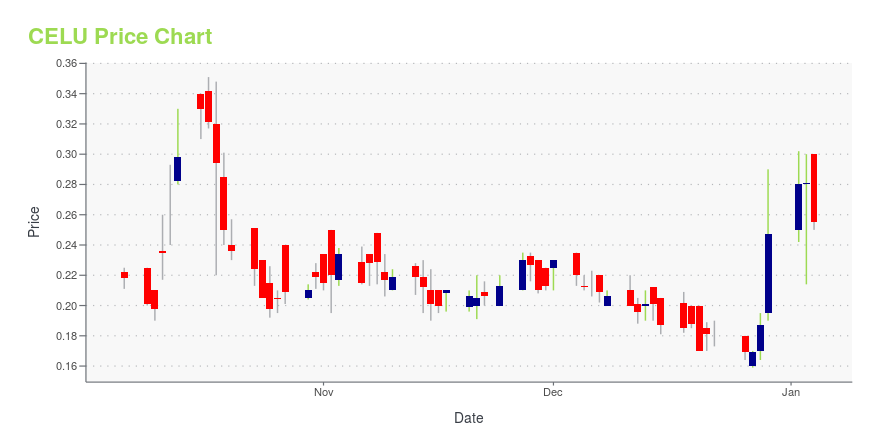
CELU Price/Volume Stats
| Current price | $3.59 | 52-week high | $5.22 |
| Prev. close | $3.74 | 52-week low | $1.00 |
| Day low | $3.51 | Volume | 109,500 |
| Day high | $3.88 | Avg. volume | 182,236 |
| 50-day MA | $2.14 | Dividend yield | N/A |
| 200-day MA | $0.00 | Market Cap | 85.98M |
CELU Stock Price Chart Interactive Chart >
Celularity Inc., (CELU) Company Bio
Celularity Inc., a clinical stage biotechnology company, develops off-the-shelf placental-derived allogeneic cell therapies. The company is developing unmodified NK cells, genetically-modified NK cells, T cells engineered with a CAR, and mesenchymal-like adherent stromal cells targeting indications across cancer, infectious diseases, and degenerative diseases. It also develops and manufactures biomaterials derived from the postpartum placenta. The company was founded in 2016 and is based in Florham Park, New Jersey.
CELU Price Returns
| 1-mo | 86.98% |
| 3-mo | 122.98% |
| 6-mo | 43.60% |
| 1-year | 19.59% |
| 3-year | -90.27% |
| 5-year | -96.48% |
| YTD | 72.60% |
| 2024 | -15.93% |
| 2023 | -80.82% |
| 2022 | -74.80% |
| 2021 | -53.45% |
| 2020 | 10.55% |

Loading social stream, please wait...