Eloxx Pharmaceuticals, Inc. (ELOX): Price and Financial Metrics
ELOX Price/Volume Stats
| Current price | $0.00 | 52-week high | $0.75 |
| Prev. close | $0.00 | 52-week low | $0.00 |
| Day low | $0.00 | Volume | 200 |
| Day high | $0.00 | Avg. volume | 1,538 |
| 50-day MA | $0.02 | Dividend yield | N/A |
| 200-day MA | $0.00 | Market Cap | 0.31K |
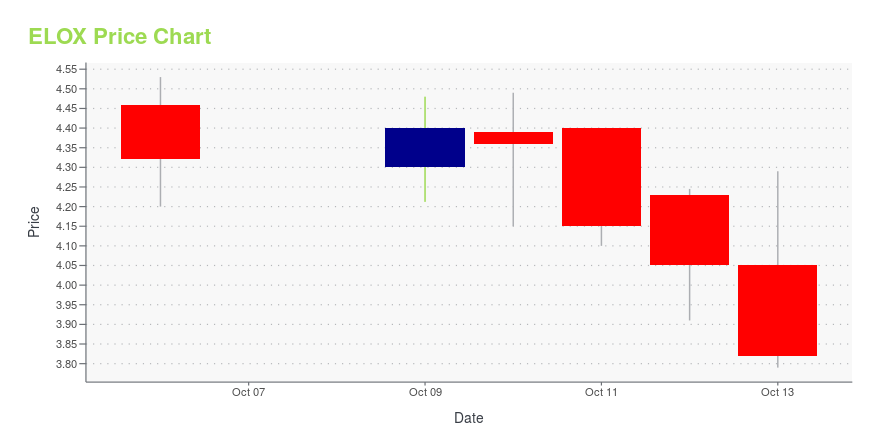
ELOX Stock Price Chart Interactive Chart >
Eloxx Pharmaceuticals, Inc. (ELOX) Company Bio
Eloxx Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, focuses on discovering and developing novel therapeutics for the treatment of rare and ultra-rare premature stop codon diseases. Its lead program is ELX-02, which is in Phase I clinical trial, which focuses on the treatment of cystic fibrosis and cystinosis patients with diagnosed nonsense mutations. The company was founded in 2013 and is based in Waltham, Massachusetts.
ELOX Price Returns
| 1-mo | N/A |
| 3-mo | N/A |
| 6-mo | N/A |
| 1-year | N/A |
| 3-year | 0.00% |
| 5-year | 0.00% |
| YTD | N/A |
| 2024 | 0.00% |
| 2023 | -34.07% |
| 2022 | -93.56% |
| 2021 | -82.26% |
| 2020 | -45.92% |

Loading social stream, please wait...