Enlivex Therapeutics Ltd. (ENLV): Price and Financial Metrics
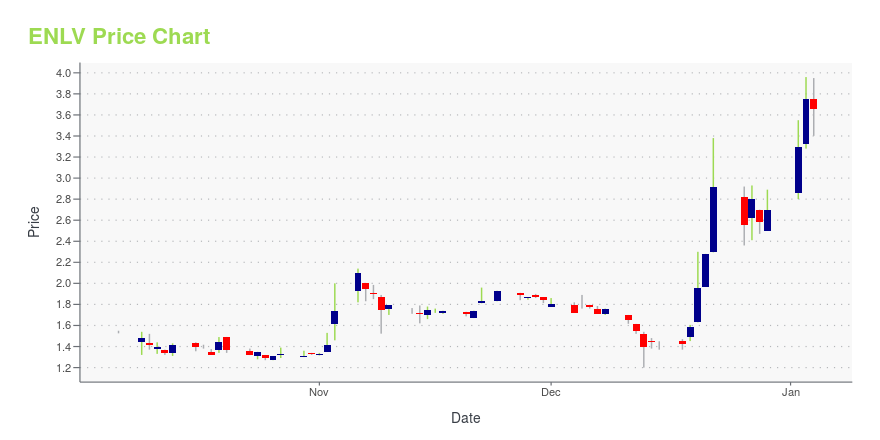
ENLV Price/Volume Stats
| Current price | $1.36 | 52-week high | $4.59 |
| Prev. close | $1.39 | 52-week low | $1.15 |
| Day low | $1.23 | Volume | 83,200 |
| Day high | $1.41 | Avg. volume | 122,217 |
| 50-day MA | $1.39 | Dividend yield | N/A |
| 200-day MA | $2.17 | Market Cap | 28.39M |
ENLV Stock Price Chart Interactive Chart >
Enlivex Therapeutics Ltd. (ENLV) Company Bio
Enlivex Therapeutics Ltd., a clinical stage immunotherapy company, engages in developing allogeneic drugs for immune system rebalancing. Its product candidate is Allocetra, an immunotherapy candidate, which is in Phase IIb clinical trial in patients with severe sepsis; that is in investigator-initiated Phase II clinical trial for the treatment of COVID-19 patients in severe and critical conditions; and which is in Phase IIa clinical trial for the prevention of Graft Versus Host Disease in allogeneic hematopoietic stem cell transplants (HSCT) patients. The company is also developing Allocetra for the prevention of complications associated with bone marrow transplantations and/or HSCT, and acute multiple organ failure. It also intends to develop its cell-based therapy to be combined with treatments of solid tumors via immune checkpoint rebalancing to enhance the efficacy of various anti-cancer therapies, including chimeric antigen receptor T-Cell therapy and therapies targeting T-Cell receptor therapy. Enlivex Therapeutics Ltd. was founded in 2005 and is headquartered in Nes Ziona, Israel.
Latest ENLV News From Around the Web
Below are the latest news stories about ENLIVEX THERAPEUTICS LTD that investors may wish to consider to help them evaluate ENLV as an investment opportunity.
Enlivex Announces Completion of Enrollment of Its Phase II Trial Evaluating Allocetra In Patients With SepsisEnrollment completed in placebo-controlled, randomized, dose-finding, multi-country, multi-center Phase II sepsis studyTopline Data Expected in late Q1 2024 Nes-Ziona, Israel, Dec. 20, 2023 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV, the “Company”), a clinical-stage macrophage reprogramming immunotherapy company, today announced that the Company completed enrollment of all 120 patients in its Phase II trial of Allocetra™ in patients with sepsis. The Phase II trial is a placebo-c |
Is Enlivex Therapeutics (NASDAQ:ENLV) In A Good Position To Deliver On Growth Plans?Just because a business does not make any money, does not mean that the stock will go down. For example, biotech and... |
MediWound Announces Appointment of Shmulik Hess, Ph.D. as Chief Operating Officer and Chief Commercial OfficerYAVNE, Israel, Nov. 21, 2023 (GLOBE NEWSWIRE) -- MediWound Ltd. (Nasdaq: MDWD), a fully-integrated biopharmaceutical company focused on next-generation enzymatic therapeutics for tissue repair, today announced the appointment of Shmulik Hess, Ph.D. to the positions of Chief Operating Officer and Chief Commercial Officer effective as of December 1, 2023. Dr. Hess will lead and oversee all operational and commercial activities at MediWound. “We are delighted to welcome Shmulik, a distinguished ind |
Enlivex to Present at the H.C. Wainwright 25th Annual Global Investment ConferenceNes-Ziona, Israel, Sept. 07, 2023 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV), a clinical-stage macrophage reprogramming immunotherapy company, today announced that company management will present a corporate update at the H.C. Wainwright 25th Annual Global Investment Conference, which will take place at the Lotte New York Palace Hotel in New York City, NY Sep 11-13. The presentation will take place on Sep 12, 2023, at 5:00 PM Eastern Time. A webcast of the presentation will be |
Enlivex Announces Peer-Reviewed Publication in Frontiers in Immunology of Clinical Data Details Resolution of Acute Respiratory Distress Syndrome (ARDS) from two Phase I/II Trials Evaluating Allocetra in Patients with COVID-19Data published in Frontiers in Immunology show a robust safety profile and rapid resolution from ARDS and parallel resolution of inflammation markers and elevated cytokines/chemokines, as well as substantial improvements in mortality in 21 Allocetra-treated patients with COVID-19 vs. matched historical controls Nes-Ziona, Israel, Aug. 02, 2023 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV, the “Company”), a clinical-stage macrophage reprogramming immunotherapy company, today announ |
ENLV Price Returns
| 1-mo | -9.33% |
| 3-mo | -5.56% |
| 6-mo | -46.46% |
| 1-year | -45.38% |
| 3-year | -82.34% |
| 5-year | -93.40% |
| YTD | -49.63% |
| 2023 | -31.43% |
| 2022 | -37.00% |
| 2021 | -25.86% |
| 2020 | 0.46% |
| 2019 | 36.22% |

Loading social stream, please wait...