GENFIT S.A. (GNFT): Price and Financial Metrics
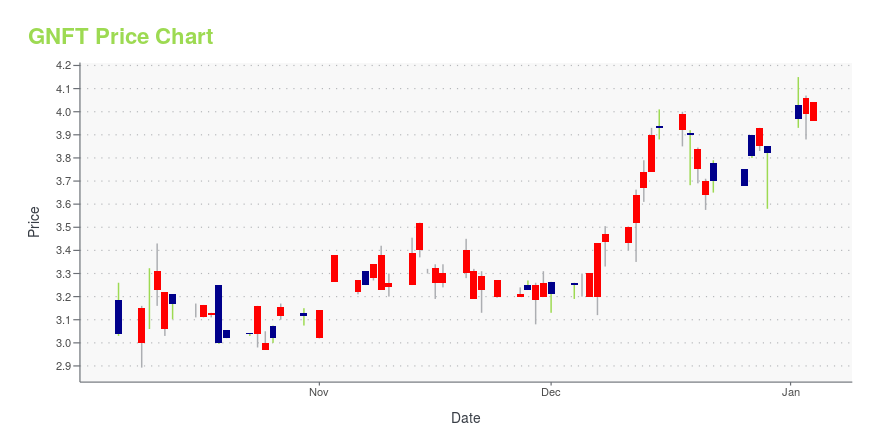
GNFT Price/Volume Stats
| Current price | $3.57 | 52-week high | $6.42 |
| Prev. close | $3.51 | 52-week low | $2.55 |
| Day low | $3.40 | Volume | 7,000 |
| Day high | $3.57 | Avg. volume | 12,019 |
| 50-day MA | $3.61 | Dividend yield | N/A |
| 200-day MA | $4.23 | Market Cap | 178.49M |
GNFT Stock Price Chart Interactive Chart >
GENFIT S.A. (GNFT) Company Bio
Genfit SA is a biopharmaceutical company involved in drug discovery and development for the early diagnosis, prevention and treatment of cardiometabolic diseases. The company focuses on the discovery and development of drug candidates in areas of high unmet medical needs corresponding to a lack of suitable treatments. It focuses on medicines to market for patients with metabolic, inflammatory, autoimmune and fibrotic diseases, that affect the liver. The company was founded by Jean-François Mouney, Florence Séjourné and Bart Staels on September 1999 and is headquartered in Loos, France.
GNFT Price Returns
| 1-mo | -1.65% |
| 3-mo | -0.83% |
| 6-mo | -36.14% |
| 1-year | -2.46% |
| 3-year | -4.80% |
| 5-year | -82.34% |
| YTD | -3.90% |
| 2024 | -3.51% |
| 2023 | -12.28% |
| 2022 | -12.22% |
| 2021 | 4.17% |
| 2020 | -75.88% |

Loading social stream, please wait...