I-Mab ADR (IMAB): Price and Financial Metrics
IMAB Price/Volume Stats
| Current price | $2.12 | 52-week high | $3.08 |
| Prev. close | $2.14 | 52-week low | $0.60 |
| Day low | $2.11 | Volume | 22,153 |
| Day high | $2.19 | Avg. volume | 424,813 |
| 50-day MA | $1.77 | Dividend yield | N/A |
| 200-day MA | $1.19 | Market Cap | 173.12M |
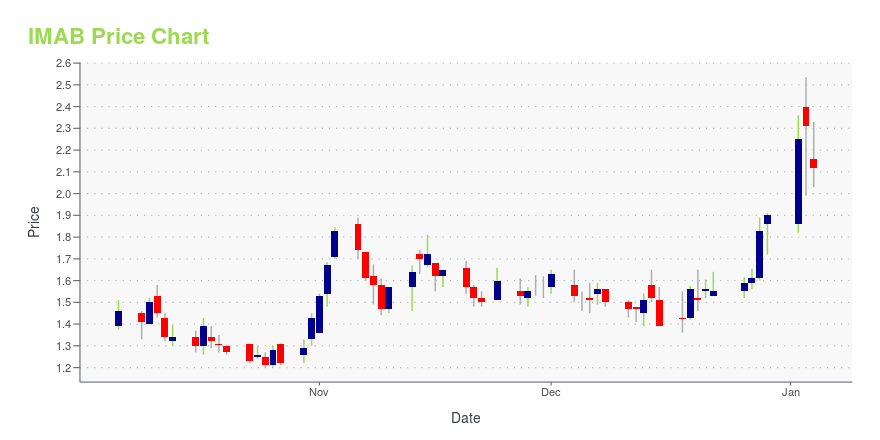
IMAB Stock Price Chart Interactive Chart >
I-Mab ADR (IMAB) Company Bio
I-Mab, a clinical stage biopharmaceutical company, engages in the discovery, development, and commercialization of novel or highly differentiated biologics to treat diseases with unmet medical needs, primarily cancers and autoimmune disorders worldwide. It is developing TJ202, a CD38 antibody in Phase III clinical trials to treat multiple myeloma and autoimmune diseases; TJ101, a long-acting human growth hormone that is in Phase II clinical trials to treat pediatric growth hormone deficiency; and TJ301, a IL-6 blocker in Phase II clinical trials for the treatment of ulcerative colitis and other autoimmune diseases. The company's product candidates in Phase I clinical trials include Enoblituzumab, a humanized B7-H3 antibody to treat immuno-oncology; TJ107, a long-acting recombinant human IL-7 to treat cancer treatment-related lymphopenia and cancer immunotherapy; TJC4, a CD47 monoclonal antibody with RBC-sparing differentiation; TJD5, a CD73 antibody for cancer treatment; and TJM2, a GM-CSF monoclonal antibody for rheumatoid arthritis and CAR-T-related therapies. Its product candidates in pre-clinical development comprise TJ210, an antibody targeting myeloid derived suppressor cells in cancers and autoimmune diseases; and TJX7, a novel CXCL13 antibody for autoimmune diseases. The company was founded in 2014 and is headquartered in Shanghai, the People's Republic of China.
IMAB Price Returns
| 1-mo | -14.86% |
| 3-mo | 151.99% |
| 6-mo | 92.73% |
| 1-year | 30.06% |
| 3-year | -76.31% |
| 5-year | -93.17% |
| YTD | 149.41% |
| 2024 | -55.26% |
| 2023 | -54.55% |
| 2022 | -91.18% |
| 2021 | 0.51% |
| 2020 | N/A |

Loading social stream, please wait...