Krystal Biotech, Inc. (KRYS): Price and Financial Metrics
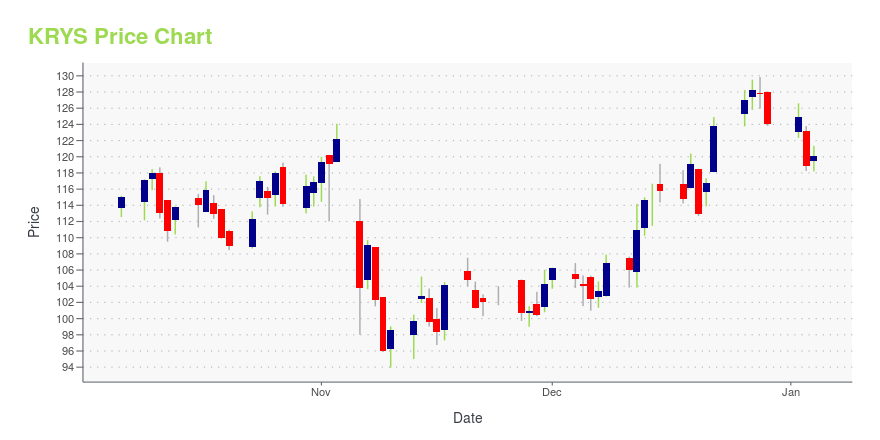
KRYS Price/Volume Stats
| Current price | $213.66 | 52-week high | $219.34 |
| Prev. close | $210.69 | 52-week low | $93.95 |
| Day low | $210.80 | Volume | 234,535 |
| Day high | $219.34 | Avg. volume | 380,332 |
| 50-day MA | $181.08 | Dividend yield | N/A |
| 200-day MA | $145.65 | Market Cap | 6.10B |
KRYS Stock Price Chart Interactive Chart >
Krystal Biotech, Inc. (KRYS) Company Bio
Krystal Biotech Inc. develops and commercializes pharmaceutical products for patients suffering from dermatological diseases. The company’s lead product candidate includes KB103, which is in preclinical studies to treat dystrophic epidermolysis bullosa, a genetic disease. The company was founded in 2015 and is based in Pittsburgh, Pennsylvania.
Latest KRYS News From Around the Web
Below are the latest news stories about KRYSTAL BIOTECH INC that investors may wish to consider to help them evaluate KRYS as an investment opportunity.
Krystal Biotech, Inc. (KRYS) Moves to Buy: Rationale Behind the UpgradeKrystal Biotech, Inc. (KRYS) has been upgraded to a Zacks Rank #2 (Buy), reflecting growing optimism about the company's earnings prospects. This might drive the stock higher in the near term. |
Krystal Biotech Receives Orphan Drug Designation from the Japanese MHLW for Beremagene Geperpavec-svdt (B-VEC)PITTSBURGH, Dec. 19, 2023 (GLOBE NEWSWIRE) -- Krystal Biotech, Inc. (the “Company”) (NASDAQ: KRYS), a commercial-stage biotechnology company focused on the discovery, development and commercialization of genetic medicines to treat diseases with high unmet medical needs, today announced that the Japan Ministry of Health, Labour and Welfare (MHLW) has granted Orphan Drug Designation (ODD) to beremagene geperpavec-svdt (B-VEC or VYJUVEK) for the treatment of dystrophic epidermolysis bullosa (DEB). |
12 Best Genomics Stocks To Buy NowIn this piece, we will take a look at the 12 best genomics stocks to buy now. If you want to skip our introduction to the genomics industry and all the latest trends, then take a look at 5 Best Genomics Stocks To Buy Now. If there’s one thing that can be said with certainty […] |
Here’s Why TimesSquare Capital Management U.S. Small Cap Growth Strategy Added Krystal Biotech (KRYS)TimesSquare Capital Management, an equity investment management company, released its “U.S. Small Cap Growth Strategy” third-quarter investor letter. A copy of the same can be downloaded here. In the quarter the fund returned -3.67% (net), compared to -7.32% return for the Russell 2000 Growth Index. Year-to-date the fund returned 11.56% (net) compared to 9.59% return for […] |
Wall Street Analysts See a 42.55% Upside in Krystal Biotech, Inc. (KRYS): Can the Stock Really Move This High?The mean of analysts' price targets for Krystal Biotech, Inc. (KRYS) points to a 42.6% upside in the stock. While this highly sought-after metric has not proven reasonably effective, strong agreement among analysts in raising earnings estimates does indicate an upside in the stock. |
KRYS Price Returns
| 1-mo | 17.27% |
| 3-mo | 36.19% |
| 6-mo | 84.14% |
| 1-year | 76.20% |
| 3-year | 274.84% |
| 5-year | 359.78% |
| YTD | 72.22% |
| 2023 | 56.60% |
| 2022 | 13.25% |
| 2021 | 16.58% |
| 2020 | 8.34% |
| 2019 | 166.51% |

Loading social stream, please wait...