Vaxcyte Inc. (PCVX): Price and Financial Metrics
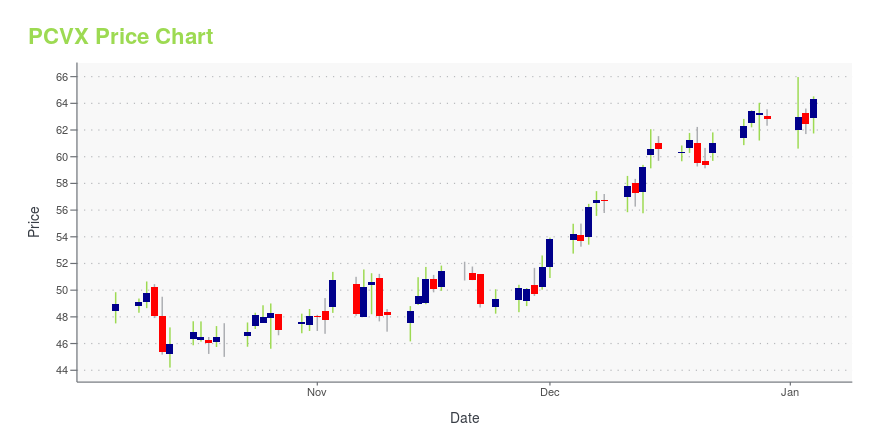
PCVX Price/Volume Stats
| Current price | $36.57 | 52-week high | $121.06 |
| Prev. close | $35.28 | 52-week low | $27.66 |
| Day low | $35.52 | Volume | 1,182,606 |
| Day high | $36.79 | Avg. volume | 1,867,614 |
| 50-day MA | $33.49 | Dividend yield | N/A |
| 200-day MA | $69.52 | Market Cap | 4.72B |
PCVX Stock Price Chart Interactive Chart >
Vaxcyte Inc. (PCVX) Company Bio
Vaxcyte, Inc. engages in development of vaccines for infectious diseases. It focuses on conjugate vaccines and protein-based vaccines developed through Sutro Biopharma's Xpress CF Platform, a cell-free protein synthesis technology. The company was founded by Grant E. Pickering, Ashish Khanna and Jeff Fairman on November 27, 2013 and is headquartered in San Francisco, CA.
PCVX Price Returns
| 1-mo | 6.52% |
| 3-mo | 19.31% |
| 6-mo | -57.13% |
| 1-year | -56.07% |
| 3-year | 46.93% |
| 5-year | 26.10% |
| YTD | -55.33% |
| 2024 | 30.35% |
| 2023 | 30.97% |
| 2022 | 101.56% |
| 2021 | -10.46% |
| 2020 | N/A |

Loading social stream, please wait...