Replimune Group, Inc. (REPL): Price and Financial Metrics
REPL Price/Volume Stats
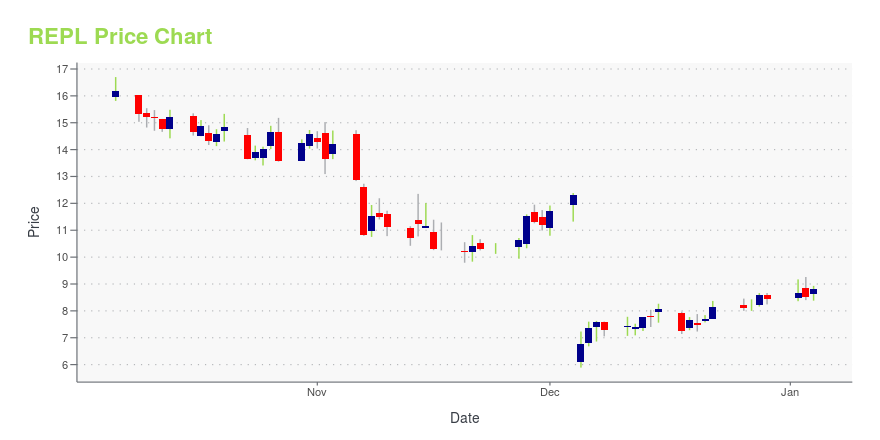
| Current price | $10.35 | 52-week high | $21.63 |
| Prev. close | $10.48 | 52-week low | $4.92 |
| Day low | $10.27 | Volume | 623,965 |
| Day high | $10.60 | Avg. volume | 1,255,832 |
| 50-day MA | $8.07 | Dividend yield | N/A |
| 200-day MA | $8.79 | Market Cap | 635.65M |
REPL Stock Price Chart Interactive Chart >
Replimune Group, Inc. (REPL) Company Bio
Replimune Ltd. develops oncolytic immunotherapies for the treatment of cancer. It is developing various proprietary products intended to enhance the direct anti-tumor effects of selective virus replication and the potency of the immune response to the tumor antigens released. The company was founded in 2015 is based in Abingdon, the United Kingdom.
Latest REPL News From Around the Web
Below are the latest news stories about REPLIMUNE GROUP INC that investors may wish to consider to help them evaluate REPL as an investment opportunity.
Replimune to Present at the 42nd Annual J.P. Morgan Healthcare ConferenceWOBURN, Mass., Dec. 28, 2023 (GLOBE NEWSWIRE) -- Replimune Group, Inc. (Nasdaq: REPL), a clinical stage biotechnology company pioneering the development of a novel class of oncolytic immunotherapies, today announced that Philip Astley-Sparke, Chief Executive Officer of Replimune, will present at the 42nd Annual J.P. Morgan Healthcare Conference on Monday, January 8, 2024 at 11:15 AM PT at the Westin St. Francis Hotel in San Francisco, CA. A simultaneous webcast will be available in the Investors |
An Intrinsic Calculation For Replimune Group, Inc. (NASDAQ:REPL) Suggests It's 24% UndervaluedKey Insights Using the 2 Stage Free Cash Flow to Equity, Replimune Group fair value estimate is US$9.64 Replimune Group... |
Why Is Replimune (REPL) Stock Down 48% Today?Replimune stock is dropping on Tuesday as investors in REPL shares react to a clinical trial missing both of its primary endpoints. |
Replimune Shares Initial Primary Analysis Results from CERPASS Clinical Trial in Advanced Cutaneous Squamous Cell Carcinoma and Presents New Data from IGNYTE Clinical Trial of RP1 in Anti-PD1 Failed Melanoma and Non-Melanoma Skin CancersRP1 in combination with cemiplimab demonstrated clinically meaningful improvements in complete response rate and duration of response compared to cemiplimab in the CERPASS clinical trial, but did not meet either of the two primary endpoints Positive data update for full 140 patients in the IGNYTE clinical trial cohort of RP1 in anti-PD1 failed melanoma reinforces durable benefit; biologics license application (BLA) submission planned for 2H 2024 RP1 monotherapy data from ARTACUS clinical trial a |
Replimune Presents Updated Data on RP2 in Uveal Melanoma during Plenary Session at the 20th International Congress of the Society for Melanoma ResearchRP2 as monotherapy and in combination with nivolumab showed a favorable safety profile and durable responses in nearly 30 percent of patients, all with previously treated diseaseWOBURN, Mass., Nov. 08, 2023 (GLOBE NEWSWIRE) -- Replimune Group, Inc. (NASDAQ: REPL), a clinical stage biotechnology company pioneering the development of a novel portfolio of oncolytic immunotherapies, today announced updated data from a cohort of metastatic uveal melanoma patients enrolled in the open-label, multicent |
REPL Price Returns
| 1-mo | 18.83% |
| 3-mo | 61.21% |
| 6-mo | 33.03% |
| 1-year | -46.87% |
| 3-year | -67.93% |
| 5-year | -20.51% |
| YTD | 22.78% |
| 2023 | -69.01% |
| 2022 | 0.37% |
| 2021 | -28.96% |
| 2020 | 165.85% |
| 2019 | 43.50% |

Loading social stream, please wait...