Cassava Sciences, Inc. (SAVA): Price and Financial Metrics
SAVA Price/Volume Stats
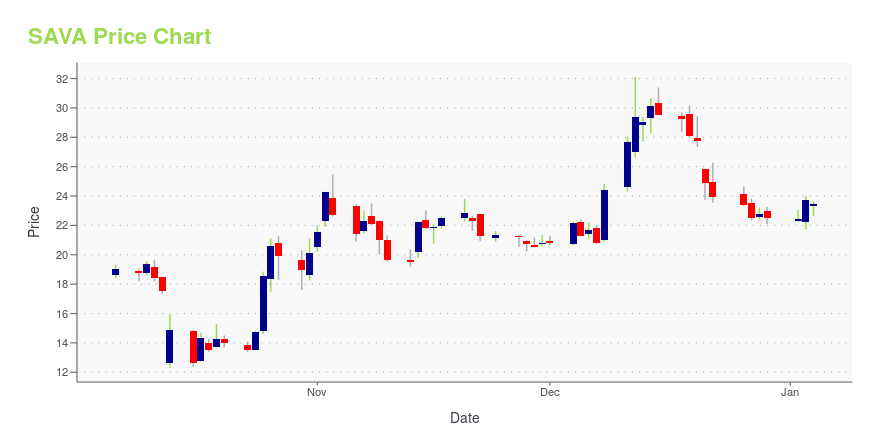
| Current price | $14.92 | 52-week high | $32.10 |
| Prev. close | $12.75 | 52-week low | $8.79 |
| Day low | $12.86 | Volume | 3,658,934 |
| Day high | $14.94 | Avg. volume | 1,335,607 |
| 50-day MA | $17.07 | Dividend yield | N/A |
| 200-day MA | $20.87 | Market Cap | 715.80M |
SAVA Stock Price Chart Interactive Chart >
Cassava Sciences, Inc. (SAVA) Company Bio
Cassava Sciences, Inc. develops drugs using its proprietary technology. The Company develops safer and efficacious drugs for use in pain management, particularly in the area of opioid painkillers. Cassava Sciences has products in clinical trials.
Latest SAVA News From Around the Web
Below are the latest news stories about CASSAVA SCIENCES INC that investors may wish to consider to help them evaluate SAVA as an investment opportunity.
3 Growth Stocks Expected to Skyrocket in 2024, According to Wall StreetWall Street analysts foresee up to 316% upside in three fast-paced companies in the new year. |
Companies Like Cassava Sciences (NASDAQ:SAVA) Are In A Position To Invest In GrowthJust because a business does not make any money, does not mean that the stock will go down. For example, although... |
Why Cassava Sciences Stock Zoomed 21% Higher This WeekThe company has chosen an offbeat way to keep its investors sweet. |
Cassava Sciences Stock Shows Rising Relative Strength Following 3 Up DaysCassava Sciences saw a welcome improvement to its Relative Strength (RS) Rating on Wednesday, with an upgrade from 79 to 88. The company earns the No. 172 rank among its peers in the Medical-Biomed/Biotech industry group. |
Why Is Cassava Sciences (SAVA) Stock Up 15% Today?Although Cassava Sciences’ issuance of warrants presents risks, the underlying Phase 3 trial support boosted SAVA stock. |
SAVA Price Returns
| 1-mo | -19.61% |
| 3-mo | -33.21% |
| 6-mo | -38.42% |
| 1-year | -26.90% |
| 3-year | -88.30% |
| 5-year | 989.05% |
| YTD | -33.72% |
| 2023 | -23.80% |
| 2022 | -32.40% |
| 2021 | 540.76% |
| 2020 | 31.15% |
| 2019 | 511.76% |

Loading social stream, please wait...