Vascular Biogenics Ltd. - Ordinary Shares (VBLT): Price and Financial Metrics
VBLT Price/Volume Stats
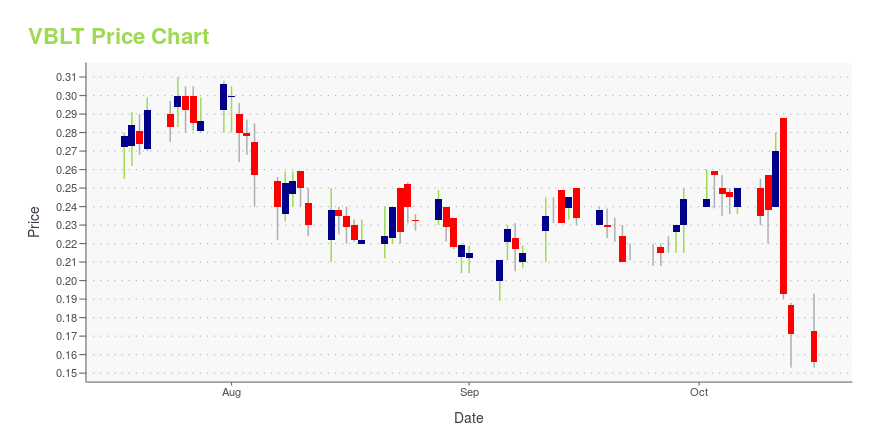
| Current price | $5.46 | 52-week high | $6.74 |
| Prev. close | $0.17 | 52-week low | $0.10 |
| Day low | $5.34 | Volume | 161,505 |
| Day high | $6.74 | Avg. volume | 2,187,886 |
| 50-day MA | $0.23 | Dividend yield | N/A |
| 200-day MA | $0.20 | Market Cap | 423.91M |
VBLT Stock Price Chart Interactive Chart >
Vascular Biogenics Ltd. - Ordinary Shares (VBLT) Company Bio
Vascular Biogenics Limited is a clinical-stage biopharmaceutical company focusing on the discovery, development, and commercialization of treatments for cancer and immune-inflammatory diseases. The company was founded in 2000 and is based in Or Yehuda, Israel.
Latest VBLT News From Around the Web
Below are the latest news stories about VASCULAR BIOGENICS LTD that investors may wish to consider to help them evaluate VBLT as an investment opportunity.
Notable Labs Closes Merger Transaction With VBL Therapeutics- Notable expects to initiate trading on the Nasdaq Capital Market under ticker symbol “NTBL” effective at market open on October 17 - - Aggregate net transaction proceeds expected to fund planned operations into 2025 - - Completed previously announced $10.3 million private placement with leading healthtech-focused investors, led by Builders VC, B Capital Group, Y Combinator, First Round Capital, and Founders Fund - - Predictive Precision Medicines Platform (“PPMP”) applied to select and develop |
VBL Therapeutics Announces Results of Annual and Special Shareholder MeetingApproved Merger with Notable Labs and All Other Proposals Merger Expected to Close and Begin Trading as Combined Company Under the NTBL Ticker on Nasdaq Next Week, Subject to Effectiveness of Approved Israeli Corporate Actions MODI’IN, Israel and NEW YORK, Oct. 12, 2023 (GLOBE NEWSWIRE) -- VBL Therapeutics (Nasdaq: VBLT) (“VBL”), today announced that its shareholders voted to approve the previously announced proposed merger (the “Merger”) with Notable Labs, Inc. (“Notable”), at the annual and sp |
VBL Therapeutics Reminds Shareholders to Vote in the Upcoming Annual and Special Shareholder MeetingShareholders of record as of September 5, 2023 are eligible to vote at the VBL Shareholder Meeting on October 12, 2023MODI’IN, Israel and NEW YORK, Oct. 04, 2023 (GLOBE NEWSWIRE) -- VBL Therapeutics (Nasdaq: VBLT) (“VBL”), reminds its shareholders to vote in favor of the previously announced proposed merger (the “Merger”) with Notable Labs (“Notable”) at the annual and special meeting (the “Meeting”) for shareholders of record as of September 5, 2023. All VBL shareholders, regardless of number o |
Today’s Biggest Pre-Market Stock Movers: 10 Top Gainers and Losers on ThursdayIt's time to start the day with a breakdown of the biggest pre-market stock movers that traders will want to watch on Thursday! |
VBL Therapeutics Announces that S-4 Registration Statement for Proposed Merger with Notable Labs Is Declared Effective by SECMerger with Notable expected to close in mid-October, subject to shareholder approval Special shareholder meeting scheduled for October 12, 2023 MODI’IN, Israel and NEW YORK, Sept. 06, 2023 (GLOBE NEWSWIRE) -- VBL Therapeutics (Nasdaq: VBLT) (“VBL”), today announced that its registration statement on Form S-4 in connection with the proposed merger with Notable Labs, Inc. (“Notable”) has been declared effective by the U.S. Securities and Exchange Commission (“SEC”). The definitive merger agreemen |
VBLT Price Returns
| 1-mo | N/A |
| 3-mo | N/A |
| 6-mo | N/A |
| 1-year | -45.26% |
| 3-year | -92.68% |
| 5-year | -88.09% |
| YTD | N/A |
| 2023 | 0.00% |
| 2022 | -93.91% |
| 2021 | 4.23% |
| 2020 | 57.50% |
| 2019 | 23.71% |
Continue Researching VBLT
Want to do more research on Vascular Biogenics Ltd's stock and its price? Try the links below:Vascular Biogenics Ltd (VBLT) Stock Price | Nasdaq
Vascular Biogenics Ltd (VBLT) Stock Quote, History and News - Yahoo Finance
Vascular Biogenics Ltd (VBLT) Stock Price and Basic Information | MarketWatch

Loading social stream, please wait...