Vanda Pharmaceuticals Inc. (VNDA): Price and Financial Metrics
VNDA Price/Volume Stats
| Current price | $6.18 | 52-week high | $6.75 |
| Prev. close | $6.15 | 52-week low | $3.30 |
| Day low | $6.10 | Volume | 357,400 |
| Day high | $6.26 | Avg. volume | 1,674,009 |
| 50-day MA | $5.71 | Dividend yield | N/A |
| 200-day MA | $4.54 | Market Cap | 359.66M |
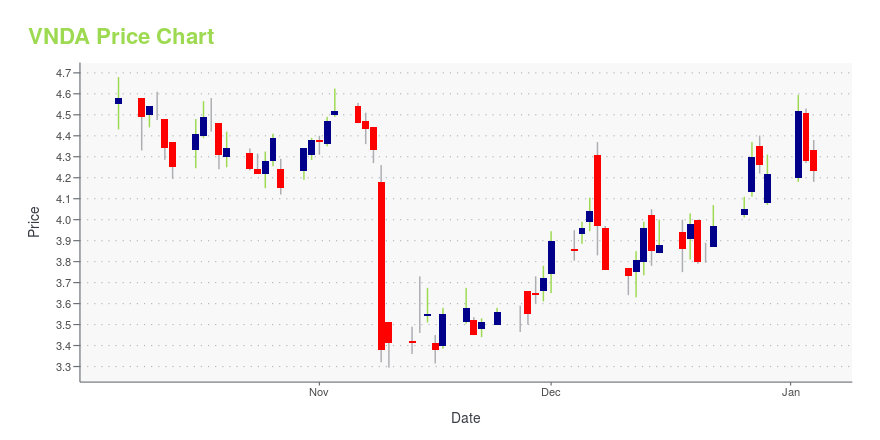
VNDA Stock Price Chart Interactive Chart >
Vanda Pharmaceuticals Inc. (VNDA) Company Bio
Vanda Pharmaceuticals is a biopharmaceutical company focused on the development and commercialization of products for the treatment of central nervous system disorders. The company was founded in 2002 and is based in Washington, the District of Columbia.
Latest VNDA News From Around the Web
Below are the latest news stories about VANDA PHARMACEUTICALS INC that investors may wish to consider to help them evaluate VNDA as an investment opportunity.
Calculating The Fair Value Of Vanda Pharmaceuticals Inc. (NASDAQ:VNDA)Key Insights Using the 2 Stage Free Cash Flow to Equity, Vanda Pharmaceuticals fair value estimate is US$4.84 Vanda... |
Vanda Pharmaceuticals Acquires U.S. and Canadian Rights to PONVORY® (ponesimod), a Selective S1P1R Modulator Approved for Patients with Relapsing Multiple SclerosisVanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today announced that it has acquired U.S. and Canadian rights to PONVORY® (ponesimod) from Actelion Pharmaceuticals Ltd. (Janssen), a Johnson & Johnson Company. PONVORY® is approved by the U.S. Food and Drug Administration (FDA) and Health Canada to treat adults with relapsing forms of multiple sclerosis (RMS), to include clinically isolated syndrome, relapsing-remitting disease and active secondary progressive disease. PONVORY® has a proven safe |
Vanda (VNDA) Rises as FDA Accepts NDA for Gastroparesis DrugThe FDA accepts Vanda's (VNDA) new drug application ("NDA") for tradipitant for treating the symptoms of gastroparesis. The company's shares rise in after-hours trading. |
Vanda Pharmaceuticals Announces that U.S. Food and Drug Administration Accepts New Drug Application for Tradipitant for the Treatment of GastroparesisVanda Pharmaceuticals Inc. (Vanda) (NASDAQ: VNDA) today announced that the U.S. Food and Drug Administration (FDA) has accepted the filing of Vanda's New Drug Application (NDA) for tradipitant for the treatment of symptoms of gastroparesis. |
One Analyst Thinks Vanda Pharmaceuticals Inc.'s (NASDAQ:VNDA) Revenues Are Under ThreatThe analyst covering Vanda Pharmaceuticals Inc. ( NASDAQ:VNDA ) delivered a dose of negativity to shareholders today... |
VNDA Price Returns
| 1-mo | 6.37% |
| 3-mo | 33.77% |
| 6-mo | 62.63% |
| 1-year | 4.04% |
| 3-year | -68.36% |
| 5-year | -50.76% |
| YTD | 46.45% |
| 2023 | -42.90% |
| 2022 | -52.90% |
| 2021 | 19.41% |
| 2020 | -19.93% |
| 2019 | -37.20% |
Continue Researching VNDA
Want to do more research on Vanda Pharmaceuticals Inc's stock and its price? Try the links below:Vanda Pharmaceuticals Inc (VNDA) Stock Price | Nasdaq
Vanda Pharmaceuticals Inc (VNDA) Stock Quote, History and News - Yahoo Finance
Vanda Pharmaceuticals Inc (VNDA) Stock Price and Basic Information | MarketWatch

Loading social stream, please wait...