Ascendis Pharma A/S (ASND): Price and Financial Metrics
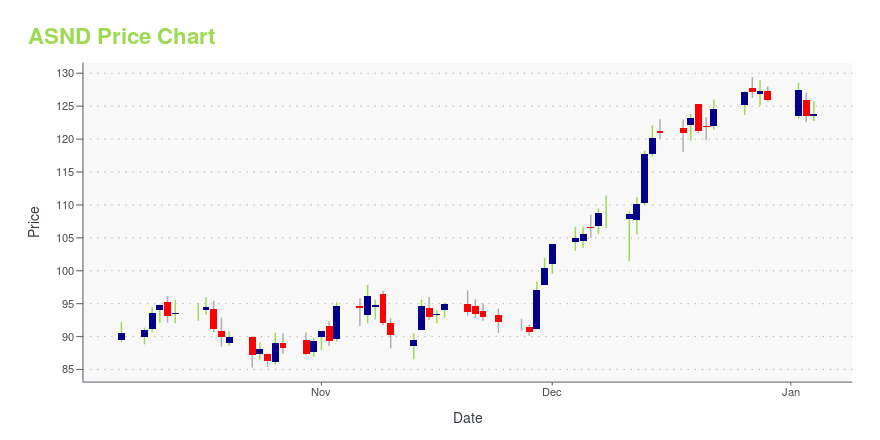
ASND Price/Volume Stats
| Current price | $136.38 | 52-week high | $161.00 |
| Prev. close | $137.74 | 52-week low | $111.09 |
| Day low | $135.61 | Volume | 690,200 |
| Day high | $141.37 | Avg. volume | 451,508 |
| 50-day MA | $129.54 | Dividend yield | N/A |
| 200-day MA | $135.11 | Market Cap | 8.27B |
ASND Stock Price Chart Interactive Chart >
Ascendis Pharma A/S (ASND) Company Bio
Ascendis Pharma A/S is applying its innovative TransCon technology, which combines the benefits of prodrug and sustained release technologies, to develop a pipeline of best-in-class therapeutics that address significant unmet medical needs. The company was founded in 2006 and is based in Hellerup, Denmark.
ASND Price Returns
| 1-mo | 11.20% |
| 3-mo | -9.84% |
| 6-mo | 5.40% |
| 1-year | 9.50% |
| 3-year | -1.91% |
| 5-year | 4.99% |
| YTD | 8.28% |
| 2023 | 3.13% |
| 2022 | -9.22% |
| 2021 | -19.34% |
| 2020 | 19.88% |
| 2019 | 122.06% |
Continue Researching ASND
Want to see what other sources are saying about Ascendis Pharma A's financials and stock price? Try the links below:Ascendis Pharma A (ASND) Stock Price | Nasdaq
Ascendis Pharma A (ASND) Stock Quote, History and News - Yahoo Finance
Ascendis Pharma A (ASND) Stock Price and Basic Information | MarketWatch

Loading social stream, please wait...