Cyclacel Pharmaceuticals, Inc. (CYCC): Price and Financial Metrics
CYCC Price/Volume Stats
| Current price | $1.51 | 52-week high | $13.20 |
| Prev. close | $1.60 | 52-week low | $1.41 |
| Day low | $1.46 | Volume | 169,251 |
| Day high | $1.56 | Avg. volume | 176,410 |
| 50-day MA | $2.27 | Dividend yield | N/A |
| 200-day MA | $4.82 | Market Cap | 1.99M |
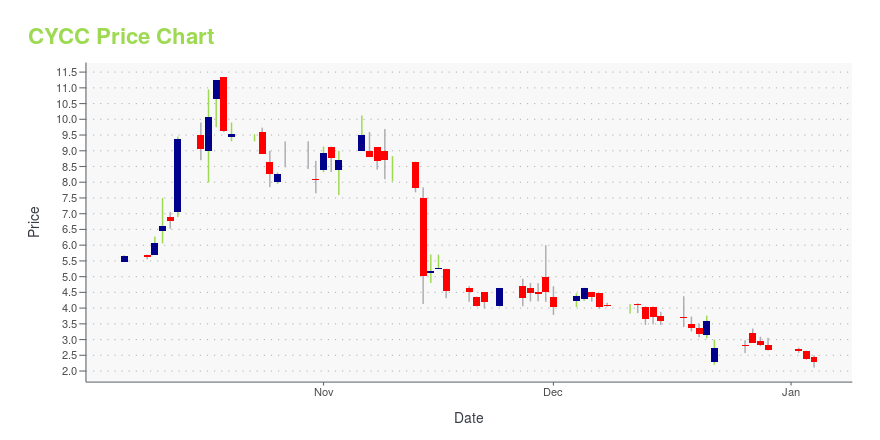
CYCC Stock Price Chart Interactive Chart >
Cyclacel Pharmaceuticals, Inc. (CYCC) Company Bio
Cyclacel Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, develops medicines for the treatment of cancer and other proliferative diseases. The company's oncology development programs include CYC065, a cyclin dependent kinase Inhibitors (CDK) that is in Phase I clinical trial for the treatment of solid tumors, as well as in combination with venetoclax to treat relapsed or refractory chronic lymphocytic leukemia; and CYC140, a polo-like kinase inhibitor program, which is in Phase I clinical trial for the treatment of advanced leukemias. Its oncology development programs also comprise sapacitabine, an oral nucleoside analogue prodrug that is in Phase 1/2 combination study with seliciclib, a CDK inhibitor in patients with BRCA mutations; with olaparib, a poly ADP-ribose polymerase inhibitor in BRCA mutation positive patients with breast cancer; and Phase III clinical trial for the treatment of acute myeloid leukemia. In addition, the company's oncology development programs include seliciclib, a CDK inhibitor that is in all-oral Phase 1/2 combination study with sapacitabine for BRCA mutations. It has a clinical collaboration agreement with the University of Texas MD Anderson Cancer Center to clinically evaluate the safety and efficacy of three cyclacel medicines in patients with hematological malignancies, including chronic lymphocytic leukemias, acute myeloid leukemias, myelodysplastic syndromes, and other advanced leukemias; and collaboration, licensing, and supply agreement with ManRos Therapeutics SA for the development and commercialization of oral seliciclib capsules for the treatment of cystic fibrosis. Cyclacel Pharmaceuticals, Inc. was founded in 1992 and is headquartered in Berkeley Heights, New Jersey.
Latest CYCC News From Around the Web
Below are the latest news stories about CYCLACEL PHARMACEUTICALS INC that investors may wish to consider to help them evaluate CYCC as an investment opportunity.
Cyclacel Pharmaceuticals Announces Registered Direct and Private Placement Offering Priced At-The-Market Under Nasdaq RulesBERKELEY HEIGHTS, N.J., Dec. 22, 2023 (GLOBE NEWSWIRE) -- Cyclacel Pharmaceuticals, Inc. (NASDAQ: CYCC, NASDAQ: CYCCP; "Cyclacel" or the "Company"), a biopharmaceutical company developing innovative medicines based on cancer cell biology, announced today that it has entered into a definitive agreement for the purchase and sale of 388,200 shares of the Company’s common stock (or pre-funded warrants in lieu thereof) at a purchase price of $3.315 per share of common stock (or pre-funded warrant in |
Cyclacel Pharmaceuticals Reports Fadraciclib Phase 1 Data Suggesting Efficacy Against Tumors With CDKN2A, CDKN2B and MTAP Deletions- Patient Pharmacodynamic Data Show Decrease in CDKN2A, CDKN2B, and PRMT5 Protein Levels - - A Squamous Non-Small Cell Lung Cancer Patient with CDKN2B Deletion Achieved Marked Tumor Shrinkage after One Cycle - - Retrospective Analysis Identified CDKN2A, CDKN2B and/or MTAP Deletions in Four Patients Previously Dosed with Fadra Achieving Responses or Stable Disease - - Forthcoming Phase 2 Study to Further Evaluate Fadra Efficacy - BERKELEY HEIGHTS, N.J., Dec. 18, 2023 (GLOBE NEWSWIRE) -- Cyclacel |
Cyclacel Pharmaceuticals Announces Reverse Stock SplitBERKELEY HEIGHTS, N.J., Dec. 12, 2023 (GLOBE NEWSWIRE) -- Cyclacel Pharmaceuticals, Inc. (NASDAQ: CYCC, NASDAQ: CYCCP; "Cyclacel" or the "Company"), a biopharmaceutical company developing innovative medicines based on cancer cell biology, today announced that it will implement a 1-for-15 Reverse Stock Split of its common stock (“Reverse Stock Split”), effective at 5:00 p.m. Eastern Time on Friday, December 15, 2023. The Reverse Stock Split, which was approved by stockholders at the Company’s Spe |
Cyclacel Regains Compliance With Nasdaq Listing Rule Following Filing of Its Form 10-Q With the SECBERKELEY HEIGHTS, N.J., Nov. 30, 2023 (GLOBE NEWSWIRE) -- Cyclacel Pharmaceuticals, Inc. (NASDAQ: CYCC, NASDAQ: CYCCP; "Cyclacel" or the "Company"), a biopharmaceutical company developing innovative medicines based on cancer cell biology, today announced that it received a notice from The Nasdaq Stock Market LLC (“Nasdaq”) indicating that the Company has regained compliance with Nasdaq Listing Rule 5250(c)(1) (the “Rule”), by filing its quarterly report on Form 10-Q for the period ended Septembe |
Health Care Roundup: Market TalkFind insights on Family Dollar, Blau Farmacêutica, Fisher & Paykel, and more in the latest Market Talks covering the Health Care sector. |
CYCC Price Returns
| 1-mo | -22.96% |
| 3-mo | -28.77% |
| 6-mo | -82.20% |
| 1-year | -82.65% |
| 3-year | -98.59% |
| 5-year | -99.28% |
| YTD | -43.45% |
| 2023 | -73.31% |
| 2022 | -82.85% |
| 2021 | -50.19% |
| 2020 | -41.72% |
| 2019 | 10.74% |

Loading social stream, please wait...