GSK PLC ADR (GSK) News
Filter GSK News Items
GSK News Results
| Date | Symbol | Company | Title | Start | End | Change | POWR Rating | ||
|---|---|---|---|---|---|---|---|---|---|
| Loading, please wait... | |||||||||
GSK News Highlights
-
404 Page not found Error: Page not found
The requested URL was not found on this server.
-
404 Page not found Error: Page not found
The requested URL was not found on this server.
-
404 Page not found Error: Page not found
The requested URL was not found on this server.
Latest GSK News From Around the Web
Below are the latest news stories about GSK PLC that investors may wish to consider to help them evaluate GSK as an investment opportunity.
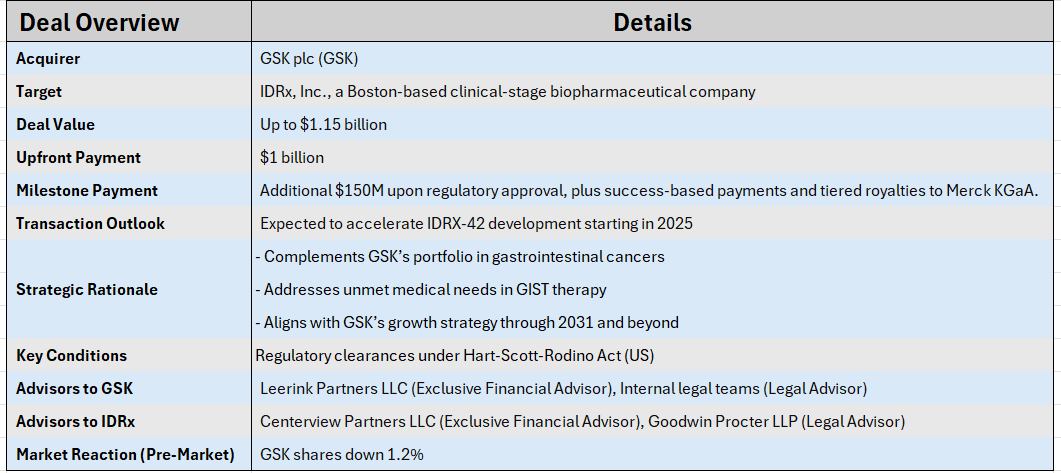
M&A Snapshot: GSK to acquire IDRx in $1.15B deal, expanding in GI cancer therapyMore on GSK |
Why Is GSK plc (NYSE:GSK) Among the Best FTSE Dividend Stocks to Buy Now?We recently compiled a list of the 10 Best FTSE Dividend Stocks To Buy Now. In this article, we are going to take a look at where GSK plc (NYSE:GSK) stands against the other FTSE dividend stocks. Goldman Sachs Research predicts moderate growth for the UK economy in 2025, with GDP rising 1.2%. That is slightly […] |
GSK’s Nucala Gains Approval in China for CRSwNPGlaxoSmithKline ( (GSK) ) has provided an update. GSK announced that its monoclonal antibody, Nucala (mepolizumab), has been approved in China for treating adults with chronic rhinosinusitis with nasal polyps (CRSwNP), a condition affecting 30 million people in the country. This approval marks the third indication for mepolizumab in China and offers patients a non-surgical treatment alternative to systemic corticosteroids and surgery. The approval is based on successful trials demonstrating its |
GSK's Sinus Disease Drug Secures Approval In ChinaOn Friday, the China National Medical Products Administration approved GSK plc (NYSE:GSK) Nucala (mepolizumab) as an add-on therapy with intranasal corticosteroids for adult patients with chronic rhinosinusitis with nasal polyps (CRSwNP) for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control. The FDA approved Nucala for the same indication in July 2021. This is the third indication for mepolizumab in China for an IL-5 mediated condition. It is estim |
Can GSK plc (LON:GSK) Maintain Its Strong Returns?While some investors are already well versed in financial metrics (hat tip), this article is for those who would like... |
GSK announces CHMP of the EMA recommended expanding approval of JemperliGSK (GSK) announced the Committee for Medicinal Products for Human Use, CHMP, of the European Medicines Agency, EMA, has recommended expanding the approval of Jemperli in combination with chemotherapy for first-line treatment of all adult patients with primary advanced or recurrent endometrial cancer who are candidates for systemic therapy. This would include patients with mismatch repair proficient /microsatellite stable tumours, who represent 70-75% of patients diagnosed with endometrial cance |
GSK announces FDA granted BTD for JemperliGSK (GSK) announced that the US Food and Drug Administration, FDA, has granted Breakthrough Therapy Designation, BTD, for Jemperli for the treatment of patients with locally advanced mismatch repair deficient/microsatellite instability-high, MSI-H, rectal cancer. The Breakthrough Therapy Designation aims to expedite the development and review of drugs with the potential to treat a serious condition and where preliminary clinical evidence may indicate substantial improvement over currently availa |
GSK’s lung cancer therapy gains EMA PRIME designationThe EMA's decision is based on the early clinical outcomes from the ARTEMIS-001 study. |
GSK's Blood Cancer Drug Shows Improved Survival Compared To Johnson & Johnson's DarzalexOn Monday, GSK plc (NYSE:GSK) announced statistically significant and clinically meaningful overall survival (OS) results from a planned interim analysis of the DREAMM-7 trial. The trial assessed Blenrep (belantamab mafodotin) in combination with bortezomib plus dexamethasone (BVd) versus Johnson & Johnson’s (NYSE:JNJ) Darzalex (daratumumab) in combination with bortezomib plus dexamethasone (DVd) as a second line or later treatment for relapsed or refractory multiple myeloma. These data were fea |
Belantamab Mafodotin shows significant overall survival benefit, reducing the risk of death by 42% in multiple myeloma at or after first relapsePHILADELPHIA, December 09, 2024--Belantamab Mafodotin shows significant OS benefit, reducing the risk of death by 42% in multiple myeloma at or after first relapse |